What Are Amino Acid Functional Groups?
 By: by Amino Science
By: by Amino Science
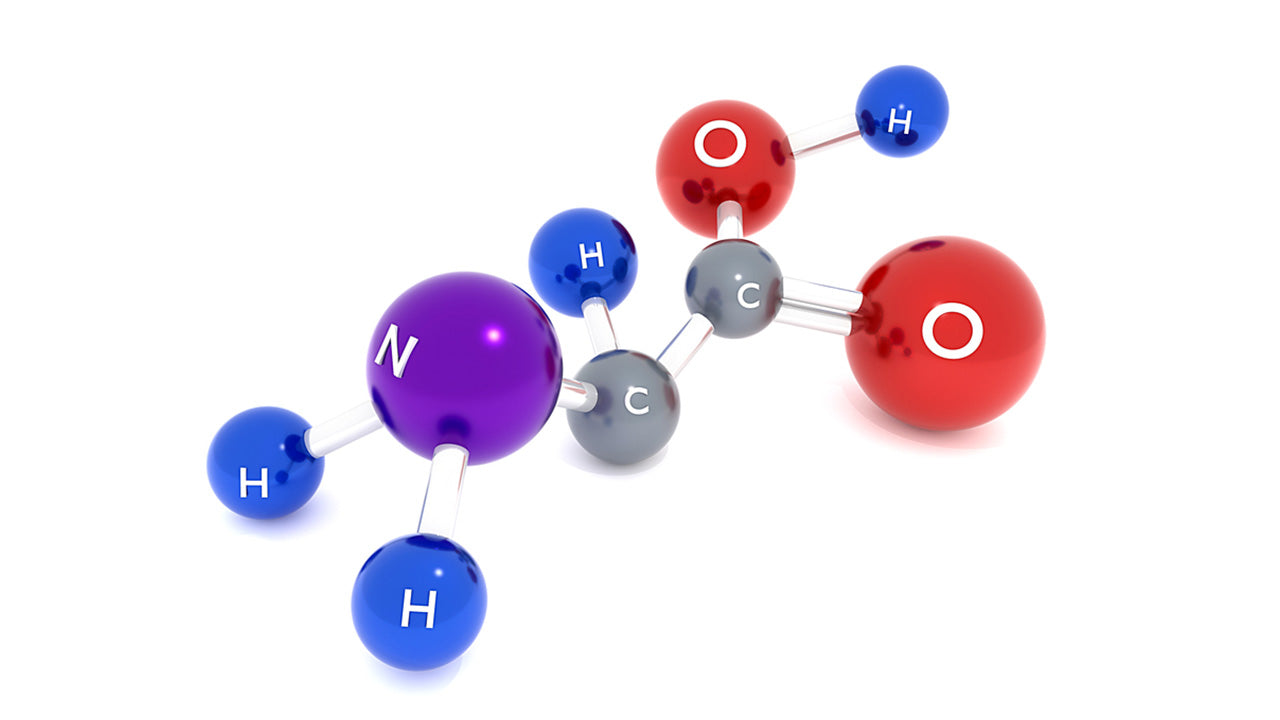
So you've heard of amino acid functional groups and you want to understand them better. This article will help to clarify the structure of amino acids, their functional groups, and what it all has to do with the creation of protein.
Some Basic Reference Definitions
Below are the names of the 20 common amino acids in the body, nine of which are essential amino acids, meaning humans must eat or otherwise consume them to get what we need to function. The essential aminos are designated with an asterisk (*). There are their names, their three-letter codes, their one-letter codes (as used in drawing models of molecular bonds), plus their bond type and properties. Polar vs. nonpolar bond types refers to their covalent bonds. We will start with those definitions for the bond type category—get ready for some high school clique metaphors to help you visualize all this organic chemistry.
Covalent Bonds
Covalent bonds form the base of the chemical reactions between atoms of different elements. The bonds form in only one way: when an electron becomes shared by two elements. That creates the connection that then results in a new substance. Covalent bonds can exist as polar or nonpolar compounds, and all bonds that are polar or nonpolar in nature are by definition covalent.
To visualize: think of these bonds like the bonds of friendship. Two students (atoms) come close to one another and share their lunch (electrons). This sharing means there is a bond of friendship (the covalent bond). Whereas before they had been two separate elements, strangers, now a new substance has formed: a friendship.
Polar Bonds
Polar bonds are bonds where the electrons are not shared equally between two atoms. These types of bonds are what designates polar molecules from nonpolar ones. This occurs when the two atoms bonding are from two different elements; atoms from the same element do not form polar bonds.
Just as bigger planets can hold bigger moons in their orbits, so it is with atoms: they pull electrons to the best of their ability, but when they come into the orbit of a better puller (a bigger planet), that atom will pull more electrons into its orbit (and steal the lesser planet's moons).
When two atoms of the same element meet, neither is stronger than the other, and thus neither one is more attractive than the other regarding electrons, and so neither side exhibits the polar pull, and the bond is considered nonpolar. The negative charge end of the bond is the more attractive atom, because it can draw more negative (-) electrons.
To put this back into the context of our high school friends, let's say the two atoms meeting and bonding are a vivacious theater student and a more reserved math student. In sharing lunch, somehow the theater student always gets more to eat because the math student is so transfixed and feels the need to be overly generous. This is true whenever the theater student meets someone with less personal magnetism, which means the theater student gets an overabundance of food (electrons), which is negative because the increased calories may cause the theater student to gain weight, or get a bad complexion from eating too much processed lunch food. Eventually the theater student will fill up to the brim with extra food, and won't be able to accept any more.
Nonpolar Bonds
A nonpolar bond is formed when two atoms from the same element meet and share electrons equally and evenly with one another. Neither atom is a better electron puller than the other, and so there is no one side more polar than the other.
In our friendship metaphor, this is a bond between two theater students, or two math students, who pool all of their lunch food together in the middle of the table, and pull from the pile equally and in unison.
Hydrophobic vs. Hydrophilic
For the properties category, hydrophobic and hydrophilic refer to molecules and their reactions to water. Molecules that repel water, or are phobic of hydration, are hydrophobic. The molecules that can form an ionic or hydrogen bond with a water molecule are hydrophilic, they have a philia for water, they like the feel of it.
If atoms are students once again, the hydrophilic ones are either on the swim team itself, or part of a group that likes to swim and heads out to the beach or the lake every weekend. The hydrophobic ones either can't swim or simply do not like it. Hydrophobic students will tell you that their friends and the community pool go together like oil and water: they don't mix.
Your Amino Acids: Names and Designations
| Amino-acid name: Alanine | Amino-acid name: Arginine |
|---|---|
| Three-letter code: Ala
One-letter code: A Bond type: Nonpolar Properties: Hydrophobic |
Three-letter code: Arg
One-letter code: R Bond type: Polar, positively charged Properties: Hydrophilic |
| Amino-acid name: Asparagine | Amino-acid name: Aspartate |
| Three-letter code: Asn
One-letter code: N Bond type: Polar, no charge Properties: Hydrophilic |
Three-letter code: Asp
One-letter code: D Bond type: Polar, negatively charged Properties: Hydrophilic |
| Amino-acid name: Cysteine | Amino-acid name: Glutamate |
| Three-letter code: Cys
One-letter code: C Bond type: Nonpolar, no charge Properties: Hydrophobic |
Three-letter code: Glu
One-letter code: E Bond type: Polar, negatively charged Properties: Hydrophilic |
| Amino-acid name: Glutamine | Amino-acid name: Glycine |
| Three-letter code: Gln
One-letter code: Q Bond type: Polar, no charge Properties: Hydrophilic |
Three-letter code: Gly
One-letter code: G Bond type: Nonpolar, no charge Properties: Hydrophobic |
| Amino-acid name: Histidine* | Amino-acid name: Isoleucine* |
| Three-letter code: His
One-letter code: H Bond type: Polar, positively charged Properties: Hydrophilic |
Three-letter code: Ile
One-letter code: I Bond type: Nonpolar Properties: Hydrophobic |
| Amino-acid name: Leucine* | Amino-acid name: Lysine* |
| Three-letter code: Leu
One-letter code: L Bond type: Nonpolar Properties: Hydrophobic |
Three-letter code: Lys
One-letter code: K Bond type: Polar, positively charged Properties: Hydrophilic |
| Amino-acid name: Methionine* | Amino-acid name: Phenylalanine |
| Three-letter code: Met
One-letter code: M Bond type: Nonpolar Properties: Hydrophobic |
Three-letter code: Phe
One-letter code: F Bond type: Nonpolar Properties: Hydrophobic |
| Amino-acid name: Proline | Amino-acid name: Serine |
| Three-letter code: Pro
One-letter code: P Bond type: Nonpolar Properties: Hydrophobic |
Three-letter code: Ser
One-letter code: S Bond type: Polar, no charge Properties: Hydrophilic |
| Amino-acid name: Threonine* | Amino-acid name: Tryptophan* |
| Three-letter code: Thr
One-letter code: T Bond type: Polar, no charge Properties: Hydrophilic |
Three-letter code: Trp
One-letter code: W Bond type: Nonpolar, no charge Properties: Hydrophobic |
| Amino-acid name: Tyrosine | Amino-acid name: Valine* |
| Three-letter code: Tyr
One-letter code: Y Bond type: Polar, no charge Properties: Hydrophobic |
Three-letter code: Val
One-letter code: V Bond type: Nonpolar Properties: Hydrophobic |
It's a lot of information, to be sure, but it's provided as a reference point to help facilitate the following explanations.
What Are Amino Acid Functional Groups?
Amino acids are all made up of groups of atoms, and the functional groups are the important ones in each amino, it's what makes them different and unique, deciding whether the amino overall is polar or nonpolar, acidic or basic.
Each of the above standard 20 aminos have one hydrogen atom, and two groups attached to its alpha carbon atom: an amino or amine group (~NH3+) and a carboxyl group (~COOH). They are each then distinguished from other types of amino acids by their third attached functional group: side chains referred to as R groups. When you ask what are amino acid functional groups, here they are, with the R group being the one with the most diversity. When you think of R groups, think R for radical, as it's the variable part that makes each amino acid different from the rest.

About R Groups
Of the 20 standard amino acids that make up the building blocks of protein:
- 6 of them have hydrocarbon R groups
- 7 of them have neutral R groups
- 6 of them have acid or base R groups
The simplest of the amino acids is glycine, which has just one hydrogen atom in the position of the side chain group (no R group at all, just an attached loner).
Some Quite Interesting Relevant Facts
- In chemistry, organic compounds are generally thought of as any chemical compound that contains carbon, which includes amino acids.
- All amino acids are soluble in an aqueous solution (water), even the hydrophobic ones (counterintuitive but true).
- All naturally occurring amino acids are in the L-form; L for levorotatory, referring to the way you'd turn the amino acid to read the order of its attached groups (the opposite of is dextrorotatory or D-form).
- As you can see in the above list, 50% of the amino acids have nonpolar side chains. The other half is designated polar, five of which have side chains that are not only polar, but charged.
- Because the carboxyl group is an acid, it can form peptide bonds with the base amino groups of other amino acids, causing chemical reactions that create polypeptide chains and amino acid residues.
- When two amino acids react and form a peptide bond, and that process goes on to be repeated, many amino acids may string together, which forms a protein structure.
- This protein synthesis (though a little more complicated than just linking acid to base over and over again like links of a chain) is among the most fundamental of biological processes, an invaluable component of every cell. Proteins make our nails and hair, protein builds and repairs our tissues, protein is needed to make our hormones, our enzymes, and many other chemicals in the human body.
- In 1953, Stanley Miller and Harold Urey conducted a simulation, to test a hypothesis about how life originally formed on Earth. They built a closed system that contained a heated pool of water under a mixture of gases that were thought to be present in the early atmosphere of Earth. They delivered an electric current to simulate lightning, and after a week analyzed the contents of the liquid pool. In there they discovered that several organic amino acids had spontaneously formed from inorganic raw materials. This leant support to the theory that the first life on Earth arose out of the primordial ooze through naturally occurring chemical reactions like a little flash of lightning.
Conclusion
The functional or R groups of amino acids are the groups that define the chemistry of proteins. They are the basis on which amino acids are classified, and according to the Miller-Urey experiment, possibly the start of all organic life on Earth. Not only is "What are amino acid functional groups?" a stimulating question to ask, but the answer to that question comes awfully close to revealing the true answers behind life, the universe, and...well...everything.
Ready to start reaping the benefits of these amino acids? Learn how they can help your health now!

Up to 25% off Amino
Shop NowTAGS: knowledge
Join the Community
Comments (0)
Most Craveable Recipes




 833-264-6620
833-264-6620